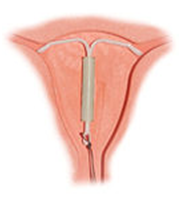
Kyleena, a hormonal contraceptive device made by Bayer AG has been approved by the U.S. Food and Drug Administration. The German company has said that this new device can last up to five years, preventing pregnancies during this time.
The intrauterine device (IUD) is T-shaped, which is small and made from flexible plastic. It works by releasing the levonorgestrel hormone to prevent the thickening of the womb lining, which consequently stops pregnancy.
A Long-lasting Solution
As of October, Kyleena will become available as one of the long-acting reversible contraceptives (LARC) on the market. These types of contraceptives have become increasingly popular again in recent years. LARCs, such as implants and IUDS are favoured instead of patches and pills, as they are more effective than these forms of contraceptives. The U.S. Center for Disease Control and Prevention has even said that their effectiveness nearly matches that of sterilisation.
Compared to the more commonly used copper IUDs (e.g. Paragard by Teva Pharmaceutical Industries Ltd.), Kyleena and other hormonal IUDs are slightly more effective. Even though the copper IUDs last longer, they don’t help to control the flow of blood.
Bayer commented that once women have been using their contraceptive for a while, it’s likely that they will experience fewer days of spotting and bleeding, whilst others may also find that their periods stop altogether.
Mirena and Skyla are two of the other hormonal IUDs that are made by Bayer, with
Kyleena being their newest one.
Other Hormonal Drugs are FDA Approved
All drug companies have to go through the FDA 510k clearance process to ensure the drugs are safe to be introduced to the public, with companies such as fdathirdpartyreview helping them to pass through this clearance process.
In February 2015, the FDA also approved another hormonal IUD. Liletta is made by Actavis Plc (Allergan Plc as they are now known) and has been approved to prevent pregnancies for a period of up to three years. This too releases the levonorgestrel hormone to prevent the womb lining from thickening and is inserted into the uterus to prevent any eggs from becoming fertilised, thus preventing pregnancies.
Whilst three years is the standard time that women use this type of contraceptive, trials have been conducted to see if Liletta can be used for as long as seven years.